Protease S and Z inhibitor genotypes in the SERPINA1 gene in patients with COPD in the Republic of Panama

Authors
DOI:
https://doi.org/10.37980/im.journal.ggcl.en.20242468Keywords:
Alpha - 1 - Antitrypsin, COPD, Alpha - 1 - Antitrypsin Deficiency, SERPINA1 , AllelesAbstract
Background: Alpha-1-Antitrypsin (AAT) is a protein that inhibits protease, especially Trypsin. AAT deficiency can cause lung diseases such as early emphysema, mainly affecting the Anglo-Saxon population; this was considered to be a rare condition in Panama. This study aimed to determine the prevalence of Alpha-1-Antitrypsin Deficiency (AATD) and allele frequency, in patients with COPD. Methods: Cross-sectional intervention study, in patients with COPD diagnosis in Panama. All patients’ AAT levels were determined with blood samples, by nephelometry. Those with AAT levels <116 mg/dL were subjected to genotyping with the real-time PCR test (AAT-mpx RealFast), which detects the protease inhibitor (PI) *M, *S y *Z of the SERPINA1 gene simultaneously. Results: 78 patients were included, 55 (70.5%) had AAT levels <116 mg/dL. The genotype distribution for these was as follows: PI*MM in 15 (27.3%), PI*MZ in 37 (67.3%), PI*MS in 2 (3.6%), and PI*SS in 1 (1,8%). Conclusion: Our findings demonstrate that AATD frequency is low in COPD patients. However, we have a high frequency of the heterozygous PI*MZ allele, which could be a future risk factor.
Methods: Cross-sectional intervention study, in patients with COPD diagnosis in Panama. All patients’ AAT levels were determined with blood samples, by nephelometry. Those with AAT levels <116 mg/dL were subjected to genotyping with the real-time PCR test (AAT-mpx RealFast), which detects the protease inhibitor (PI) *M, *S y *Z of the SERPINA1 gene simultaneously.
Results: 78 patients were included, 55 (70.5%) had AAT levels <116 mg/dL. The genotype distribution for these was as follows: PI*MM in 15 (27.3%), PI*MZ in 37 (67.3%), PI*MS in 2 (3.6%), and PI*SS in 1 (1,8%).
Conclusion: Our findings demonstrate that AATD frequency is low in COPD patients. However, we have a high frequency of the heterozygous PI*MZ allele, which could be a future risk factor.
INTRODUCCION
Alpha 1-antitrypsin (AAT) is a 52 kD glycoprotein, mainly synthesized in the liver, with a half-life in the blood of approximately 5 days. It was recently 50 years since the first description of alpha 1-antitrypsin deficiency (AATD) by Laurell and Eriksson, who observed the absence of the alpha 1 band of globulins in electrophoresis. The set of variants is called Pi (protease inhibitor) system, the variants were assigned with the letter of the alphabet according to the electrophoretic migration of the gel, they called Pi*M to the average speeds, Pi*S slow migration and Pi*Z to the very slow migration, from there arose more investigations which the normal allele, present in more than 85% of the individuals, is denominated M and the most frequent mutants S and Z. Today, heterozygous variants are the most common variants already described. Its main function is to inhibit neutrophilic elastase (NE) in the lung, thus protecting lung tissue from proteolytic degradation. Alpha 1-antitrypsin deficiency (AATD) is an autosomal co-dominant disorder that occurs most frequently in adulthood. AATD is caused by variants in the SERPINA1 gene, located on the long arm of chromosome 14 (14q32.1). The most common variants are the Z and S variants, caused by the substitution of glutamic acid for lysine or valine at positions 342 and 264 of the polypeptide, respectively.
More than 120 variants have been identified to date, but the most common are Pi*Z and Pi*S. Carriers of AATD are at increased risk for early emphysema and later chronic obstructive pulmonary disease (COPD) [6]. Despite scarce epidemiological information worldwide, several studies have shown that AATD is relatively common among Europeans, and its frequency may be 2,000 to 4,000 persons [7, 8, 9].
According to studies, patients with AATD may experience significant airflow limitation without airway obstruction, and it is often greater than their smoking history. Only 15-30% of smokers experience COPD [10,11]. Risk exposures may also affect lung function in patients with AATD found in COPD patients (smoking, environmental exposures, biomass exposure, age, and baseline lung function) [11].
Inherent problems include the difficulty in understanding genetic variants and their relevance; new approaches are needed in the design and implementation of clinical trials, such as gene silencing strategies. The fact that the Panamanian population is ethnically diverse, including immigrants from European countries where the frequency of alleles implicated in early pulmonary changes is high, suggests that AATD is underdiagnosed in Panama. Although the calculated prevalence for COPD in Panama is 4.4% [12], the prevalence of AATD in this population is still unknown and for years it was not considered to be affecting our population. We conducted this study with the intention of knowing the prevalence of AATD, as well as the allele frequency, in patients diagnosed with COPD in Panama.
METHODS
Study design
A cross-sectional intervention study was carried out in patients with a previous diagnosis of COPD, who were under follow-up in the pulmonology department of the Hospital Dr. Arnulfo Arias Madrid; this is a national referral general hospital, belonging to the Caja de Seguro Social, located in Panama City. The study was evaluated, approved and registered by the Ethics Committee of the Hospital Dr. Arnulfo Arias Madrid, under number CBI - CHDrAAM - 141 - 2022.
Patients
Patients over 18 years of age, with an established diagnosis of COPD, by clinical evaluation and simple and bronchodilator spirometry, performed by a pulmonology specialist, were included. Patients were recruited in the outpatient section of the hospital's pulmonology department.
Patients over 18 years of age and with a clinical diagnosis of COPD/AA enzyme deficiency were included and oncologic patients were excluded, as well as those with immunosuppression due to medication or other causes, acute infectious process and any condition that could hinder the comprehension of the study, either due to neurological or psychiatric disease, or language barriers.
After informed consent, the clinical record was questioned and checked for age, sex, ethnicity, province of origin, and risk exposure (smoking, biomass, or other environmental or occupational exposures).
Determination of serum alpha 1-antitrypsin (AAT) concentration
Ten ml of venous blood was obtained and distributed as follows: 4 ml of whole blood and 6 ml of serum. An initial determination of serum AAT levels was performed on this sample. AAT levels were determined via the Siemens nephelometry technique. Internal test controls were used to validate the AAT values measured in serum, and a cut-off point for normal AAT values (116 - 220 mg/dL) was considered. Subsequently, in case serum AAT levels < 116 mg/dL were found, genotyping was performed (Figure 1).
DNA isolation and AAT genotyping
EDTA-extracted whole blood was used for DNA extraction; DNA was extracted with a NucleoSpin MACHEREY - NAGEL blood DNA extraction kit [13], following the manufacturer's instructions. For the identification of the S and Z alleles in exons 3 and 5, in the 145 bp and 153 bp fragments of the SERPINA1 gene, respectively, SNP genotyping tests by real-time PCR AAT mpx RealFastTM Assay (ViennaLab Diagnostics) [14] were used, which was fast and accurate for the simultaneous detection of *S and *Z variants of the protease inhibitor (PI). In addition, controls for the normal genotype (WT - Control) and the homozygous mutant (MUT - Control) were used.
Statistical analysis
A descriptive statistical analysis was performed. The mean or median, with its dispersion value for quantitative variables, and the absolute and relative frequencies for quantitative variables were calculated.
Differences between categorical variables between the variants and the variables of sex, age, origin and smoking were calculated using the x2 (chi - square) for the crossover between two qualitative variables [15].
RESULTS
Seventy-eight patients with diagnosed COPD were included. AAT lower than 116 mg/dl was found in 55 patients, who underwent genotyping (Figure 1).
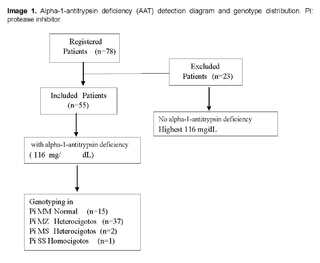
The characteristics of the 55 patients are shown in (Table 1). The median age was 65 years with a standard deviation of ±15.5, 56.4% were male.
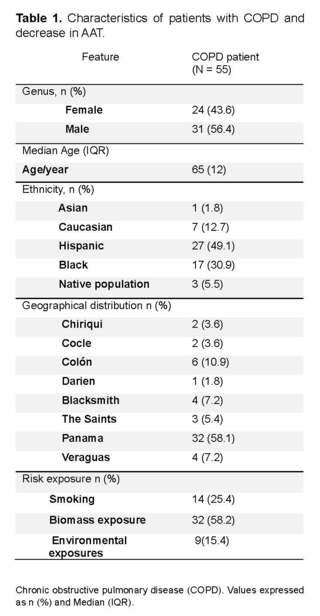
Forty-nine.1% identified themselves as Hispanic and 30.9% as black. The 58.1% came from the province of Panama, followed by the province of Colon with 10.9%. Regarding risk exposure, 58.2% reported exposure to biomass and 25.4% reported a history of active smoking (Table 1).
The genotype distribution was as follows: Pi*MM with 27.3% (15/55) of patients, Pi*MZ allele with 67.3% (37/55), Pi*MS with 3.6% (2/55) and Pi*SS with 1.8% of the total sample (1/55).
Demographic characteristics stratified by genotype are shown in (Table 2). In the study, the overall prevalence of HAD in the Panamanian population was 0.7%, and the genotype with the highest prevalence was Pi*MZ. When analyzing the characteristics of sex, age and ethnicity, we observed that patients with deficiency were characterized by a tendency towards the Pi*MZ genotype, compared to patients with mild or non-deficient genotype (Pi*MM respectively) (p = > 0.005).
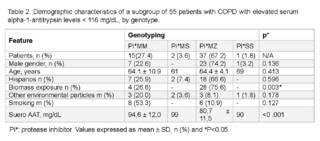
After adjusting for the associated variables of risk exposures, independently of genotype, it could be observed that those exposed to biomasses were more likely to have the Pi*MZ genotype (75.6%, p = 0.003). However, there was no difference in environmental exposure and smoking history between the genotypes of the patients.
In patients with available results, serum AAT levels were below the treatable range for the observed genotypes compared to patients without the disease (p = < 0.001).
DISCUSSION
The prevalence of COPD has shown an increase worldwide, but in the national prevalence study in Panama, one of the lowest prevalences in the region was reported, with 4.4% (CI 3.1- 5.8) [16, 17].
The global risk factors for COPD continue to be environmental factors, especially exposure to tobacco; in Latin American countries, exposure to biomass, such as particulate matter from smoke produced by burning biofuels, such as wood, animal manure or crop residues, remains an important risk factor. The most frequent generic factor for developing the disease is alpha 1-antitrypsin AATD deficiency [18], which has a low prevalence.
In this study, we sought to identify the most frequent deficiencies and genotypes in our population. To detect deficiency, we used the serum AAT value of 116 mg/dl, which corresponded to the maximum value of the confidence interval range, measured by nephelometry [19]; this was done to identify patients with severe AATD, as well as patients with moderate and mild AATD.
Worldwide, the prevalence of AATD is estimated to be between 0.06% in East Asia and 7.4% in Southern European countries [20, 21]. In our study, we found a prevalence of 0.7%, which is within low ranges, and is similar to other countries in the region; Brazil has a prevalence of 2.8%, mainly of the Pi*ZZ genotype, Argentina has a prevalence of 1.29%, mainly of the Pi*ZZ genotype [22], and Colombia has a prevalence of 0.69%, mainly of the Pi*MS genotype [23].
In contrast to other Latin American studies, in which the most common genotypes were Pi*ZZ and Pi*ZS, our case corresponded to a patient with the Pi*SS genotype [16, 16, 19]. In addition, the genotype we found most frequently was the heterozygous Pi*MZ, which corresponds to the most frequent variant worldwide, about 20%. In our case, it was 67.2%, which is much higher than what has been reported worldwide. These were mainly associated with patients exposed to biomass, which could represent a risk for a future increase in cases.
The Pi*MZ heterozygous population is described to have an increased risk of impaired lung function and an increased risk of severe COPD. However, no conclusions have been reached on the usefulness or benefit of specific treatment. Heterozygous variants have also been reported to be associated with other extrapulmonary diseases, such as liver disease.
It is important to note that biomass exposure is considered the second leading cause of COPD, after smoking. In this study, we compared whether patient age, gender, and ethnicity were associated with the variants. We found no significant differences in the variables; this is probably because the severity of the disease does not significantly affect the variables, and instead depends largely on the high risk of exposure one may have.
This was the first study performed in COPD patients to evaluate alpha 1-antitrypsin deficiency and its genotypes in the Republic of Panama. All the data supporting the findings of this study are available in the article. Additional information is available upon reasonable request to the corresponding author. It shows the presence of heterozygous genotypes in 2/3 of the studied population, which is a significant contribution to understand this disease in our country.
The main limitation of the study was that it was carried out in patients with social security, received in a hospital in the capital city, which does not represent the total population of the country. For this reason, it is recommended to extend the analysis to populations throughout the country with variable environmental exposures, and with ethnicities such as native indigenous peoples in order to have a more complete understanding of our country. It would be valuable to study the relationship between lung function and liver function in Pi*MZ heterozygous patients, which were the most frequent in our country.
CONCLUSION
The prevalence of alpha 1-antitrypsin in Panama City is low and similar to that reported in the region. The most frequent homozygous genotype was Pi*SS, and the heterozygous genotype was Pi*MZ, which was more associated with biomass exposure. Therefore, new strategies are needed to improve detection, especially, because the available evidence supports the clinical efficacy of augmentation therapy and new alternative therapies are currently being investigated.
Acknowledgments
Thanks to Scientific Instruments S.A, for their contribution of NucleoSpin blood DNA and real AAT mpx RealFastTM ViennaLab Diagnostics kits. To the NUX Laboratory for the support in their facilities. To the Pneumology Service of the Hospital Dr. Arnulfo Arias Madrid and the Caja de Seguro Social for the enrollment of COPD patients. To the genetics laboratory of the Hospital Dr. Arnulfo Arias Madrid and the Caja de Seguro Social for their technical support in the performance of the laboratory measurements. The research work was presented as research work for the master's degree in molecular biology at the University of Panama 2024, poster presentation at the First Iberoamerican Congress of Medical Genetics and Genomic Medicine 2023 and at the congress of the Latin American Association of Thorax (ALAT) 2024.
Authors' contributions
LDG: Conceptualization and construction of the research protocol, sample collection and analysis, data analysis, drafting of the manuscript.
LN: Review and revision of protocol, enrollment of COPD patients, review of data analysis, correction of manuscript, revision and drafting.
ES: Standardization of tests and specimen analysis.
LS: Protocol review and revision.
AS: Research protocol conceptualization and editing.
OE: Conceptualization and writing of the research article and data analysis.
References
Hatipoğlu U, Stoller JK. α1-Antitrypsin deficiency. Clin Chest Med. 2016;37(3):487–504. https://doi.org/10.1016/j.ccm.2016.04.011
Vidal R, Blanco I, Casas F, Jardí R, Miravitlles M. Diagnóstico y tratamiento del déficit de alfa-1-antitripsina. Arch Bronconeumol. 2006;42:645–59. https://doi.org/10.1157/13095974
Janoff A. Elastases and emphysema: Current assessment of the protease-antiprotease hypothesis. Am Rev Respir Dis. 1985;132:417–33.
Falfán R, Silva I, Pérez G, Camarena A, Morales F, Montaño M, et al. Bases genéticas y moleculares de alfa-1 antitripsina (SERPINA1) y su papel en la EPOC. Rev Inst Nal Enf Resp Mex. 2009;22(2):124–36.
Stockley RA. The pathogenesis of chronic obstructive lung diseases: implications for therapy. QJM. 1995;141–6.
Martínez Luna M, Rojas Granados A, Lázaro Pacheco RI, Meza Alvarado JI. Enfermedad pulmonar obstructiva crónica (EPOC). Rev Fac Med (Mex). 2021;63(3):28–35. https://doi.org/10.22201/fm.24484865e.2020.63.3.06
Blanco I, de Serres FJ. Números estimados y prevalencia de los alelos PIS y PIZ de la deficiencia de alfa1-antitripsina en países europeos. Eur Respir J. 2006;77–84.
de Serres F, Blanco I. Papel de la alfa-1 antitripsina en la salud y la enfermedad humanas. Rev Med Interna Blackwell Publ Ltd. 2014;12239. https://doi.org/10.1111/joim.12239
Blanco I, Bueno P, Diego I, et al. Frecuencia del gen alfa-1 antitripsina PiZ y números del genotipo PiZZ en todo el mundo: una actualización. Int J Chron Obstruct Pulmon Dis. 2017;12:561–9. https://doi.org/10.2147/COPD.S125389
Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary. Eur Respir J. 2004;23:932–46. https://doi.org/10.1183/09031936.04.00014304
Needham M, Stockley RA. α1-Antitrypsin deficiency: Clinical manifestations and natural history. Thorax. 2004;59:441–5. https://doi.org/10.1136/thx.2003.006510
Noriega-Aguirre LI, Méndez J, Trujillo A. Prevalence and characteristics of chronic obstructive pulmonary disease in Panama Republic. Neumol Cir Torax. 2021.
Gillespie D, Vogelstein B. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci USA. 1979;76:615–9. https://doi.org/10.1073/pnas.76.2.615
ViennaLab Diagnostics GmbH. AAT mpx RealFast™ Assay. Vienna, Austria; 2020.
Menga G, Fernandez Acquier M, Echazarreta AL, Sorroche PB, Lorenzon MV, Fernández ME, et al. Prevalencia de déficit de alfa-1 antitripsina en pacientes con EPOC en Argentina. Arch Bronconeumol. 2020;571–7. https://doi.org/10.1016/j.arbres.2019.11.010
Noriega-Aguirre LI, Méndez J, Trujillo A. Prevalence and characteristics of chronic obstructive pulmonary disease in Panama Republic. Neumol Cir Torax. 2021.
López Varela MV, Muiño A, Pérez Padilla R, Jardim JR, Tálamo C, Montes de Oca M, et al. Tratamiento de la EPOC en 5 ciudades de América Latina: estudio PLATINO. Arch Bronconeumol. 2008;44:58–64. https://doi.org/10.1157/13115743
American Thoracic Society/European Respiratory Society. Estándares para el diagnóstico y el tratamiento de personas con deficiencia de alfa-1 antitripsina. Am J Respir Crit Care Med. 2003;168(7):818–900. https://doi.org/10.1164/rccm.168.7.818
Stoller JK, Aboussouan LS. Una revisión de la deficiencia de α1-antitripsina. Am J Respir Crit Care Med. 2012;185(3):246–59. https://doi.org/10.1164/rccm.201108-1428CI
Russo R, Zillmer LR, Nascimento OA, Manzano B, Ivanaga IT, Fritscher L, et al. Prevalence of alpha-1 antitrypsin deficiency and allele frequency in patients with COPD in Brazil. J Bras Pneumol. 2016;42(5):311–6. https://doi.org/10.1590/S1806-37562015000000180
Luisetti M, Massi G, Massobrio M, Guarraci P, Menchicchi FM, Beccaria M, et al. A national program for detection of alpha 1-antitrypsin deficiency in Italy. Respir Med. 1999;169–72. https://doi.org/10.1016/S0954-6111(99)90003-3
Menga G, Acquier MF, Echazarreta AL, Sorroche PB, Lorenzon MV, Fernández ME, et al. Prevalencia de deficiencia de Alfa-1 antitripsina en pacientes con EPOC en Argentina. Arch Bronconeumol. 2020;56(9). https://doi.org/10.1016/j.arbres.2019.11.010
Alí-Munive A, Leidy P, Proaños NJ, Pedrozo-Pupo J, Giraldo A, Cano D, et al. Prevalence of genetic mutations in alpha-1 antitrypsin deficiency (AATD) in patients with COPD in Colombia. BMC Pulm Med. 2023;23(1):156. https://doi.org/10.1186/s12890-023-02453-0
Strange C, Ashry HS. COPD in individuals with the Pi*MZ alpha-1 antitrypsin genotype. Eur Respir Rev. 2017;26:146. https://doi.org/10.1183/16000617.0068-2017
Calero Acuña C, López Ramírez C, Represas Represas C, Priegue Carrera A, Casamor R, Fernández Villar A, et al. Evaluación de la exposición a factores de riesgo alternativos al tabaco en la EPOC en Neumosur. Rev Esp Patol Torac. 2015;27:195–200.
Stoller JK, Aboussouan LS. Deficiencia de alfa1-antitripsina. Lancet. 2005;2225–36.
Marmerpaul D, Hurtubise E. Nephelometric and turbidimetric immunoassay. In: Immunoassay. 1996. p. 363–87. https://doi.org/10.1016/B978-012214730-2/50018-2
Alí-Munive A, Leidy P, Proaños NJ, Pedrozo-Pupo J, Giraldo A, Cano D, et al. Prevalence of genetic mutations in alpha-1 antitrypsin deficiency (AATD) in patients with COPD in Colombia. BMC Pulm Med. 2023;23(1):156. https://doi.org/10.1186/s12890-023-02453-0
Dahl M, Tybjaerg-Hansen A, Lange P, Vestbo J, Nordestgaard BG. Cambio en la función pulmonar y la morbilidad por EPOC en heterocigotos para alfa1-antitripsina MZ: un estudio longitudinal. Ann Intern Med. 2002;136(4):270–9.
Bartels CL, Marchetti AL, Highsmith EW, Tsongalis GJ. Detección por PCR en tiempo real de las variantes PIZ y PIS asociadas con la deficiencia de alfa-1 antitripsina. Am J Transl Res. 2009;1(4):406–11.
Vidal R, Blanco I, Casas F, Jardí R, Miravitlles M. Guía para el diagnóstico y manejo del déficit de alfa-1 antitripsina. Arch Bronconeumol. 2006;42(12):645–59. https://doi.org/10.1157/13095974
Blanco I, de Serres FJ, Cárcaba V, Lara B, Fernández-Bustillo E. Distribución de frecuencias de los genes PIZ y PIS utilizando mapas mundiales. Hepat Mon. 2012;12(10):e7434.
García-Palenzuela R, Timiraos Carrasco R, Gómez-Besteiro MI, Lavia G, Lago Pose M, Lara B. Detection of alpha-1 antitrypsin deficiency in COPD patients in primary care. Arch Bronconeumol. 2017;43:289–94
suscripcion
issnes
eISSN L 3072-9610 (English)
Information
Use of data and Privacy - Digital Adversiting
Infomedic International 2006-2025.

