Prevalence of Metabolic Syndrome X in patients diagnosed with Chronic Kidney Disease undergoing dialysis


Authors
DOI:
https://doi.org/10.37980/im.journal.ggcl.en.20252691Keywords:
Metabolic Syndrome X, abdominal obesityinsulin resistance, chronic kidney disease, geneticsAbstract
Introduction: Metabolic syndrome X (MSX) encompasses risk factors such as abdominal obesity, hypertension, dyslipidemia, and insulin resistance, which significantly increase cardiovascular morbidity and mortality. In patients with chronic kidney disease (CKD), the presence of MSX is even more relevant, as it accelerates disease progression and increases cardiovascular complications. A retrospective, descriptive study was conducted in which patients treated at the Hemodialysis Unit of Dr. Amador Guerrero Hospital were evaluated, and the presence of cardiovascular risk factors and components of MSX was quantified. Justification: Metabolic syndrome is highly prevalent in patients with chronic kidney disease and increases cardiovascular risk, making its identification essential to improve clinical outcomes. Methodology: Patients with chronic kidney disease undergoing dialysis in the province of Colón during 2005 were studied. Anthropometric and laboratory data were collected, and metabolic syndrome was diagnosed according to the NCEP/ATP III (2005) criteria. Data analysis was performed using EPI-INFO 6.0. Results: A prevalence of 23.7% of MSX was observed among patients treated at the unit. Of these patients, 88.9% presented abdominal obesity and arterial hypertension. However, only abdominal obesity and insulin resistance (measured as fasting hyperglycemia) were statistically significant for the presence of MSX in CKD patients, with reported ORs of 38.4 (95% CI: 3.88–379.70, p<0.05) and 6.28 (95% CI: 1.23–31.95, p<0.05), respectively. Conclusion: The prevalence of MSX in the studied population was 23.7%. Abdominal obesity and insulin resistance significantly increased the likelihood of MSX. Furthermore, current evidence suggests that genetic variants may predispose CKD patients to the development of MSX, opening new avenues for research.
INTRODUCTION
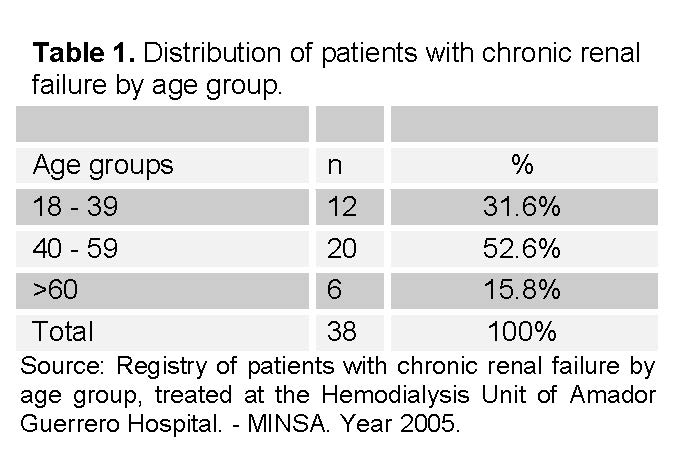
In the years prior to 2005, there was a significant increase in the prevalence of patients with chronic renal failure (CRF). In the province of Colón, a total of 53 patients were identified in the hemodialysis and peritoneal dialysis programs of the Hemodialysis Unit at Manuel Amador Guerrero Hospital. This trend of increasing cases was observed at this facility and in international reports with an alarming trend [1].
In the context of chronic renal failure (CRF), it is essential to recognize that diabetes mellitus (DM) and hypertension (HTN) are the main comorbidities and, at the same time, represent direct and well-established causes of this disease. On the other hand, dyslipidemia is considered more of a frequent consequence of CRF and a factor that contributes to its progression [1], but not a primary cause of it. However, the concurrence of DM and HTN along with dyslipidemia, insulin resistance, and central obesity constitutes the so-called metabolic syndrome (MSX), which is closely associated with the onset and worsening of CRF [2,3].
According to studies, four factors contribute 79% or more to the development of MSX [4]: insulinemia, body mass index, blood pressure, and lipid levels, with insulinemia being the most related (IRR 1.81, p<0.01), followed by body mass (IRR 1.52, p<0.01) and lipids (IRR 1.37, p<0.01), while blood pressure showed no significant association (IRR 1.11, p=0.20) [2]. In population studies such as NHANES 1999-2000, the reported prevalence of MSX was 27.6% [3].
During the year of this study, the population of Colón consisted of 219,970 people, of which 58% resided in the district of Colón. Given the prevalence of multiple comorbidities observed in this population, it was decided to conduct this study on factors associated with Metabolic Syndrome X in hemodialysis patients in this city [5,6].
We also conducted a review of the genetic aspects that may contribute to the predisposition to Metabolic Syndrome X and Chronic Renal Failure. Polymorphisms in genes such as LEP, ADIPOQ, PPARG, INSR, IRS1, APOE, LPL, ACE, and TNF have been associated with abdominal obesity, insulin resistance, dyslipidemia, hypertension, and chronic inflammation [7,8,9]. This knowledge helps to understand the clinical heterogeneity and paves the way for personalized preventive strategies.
METHODOLOGY
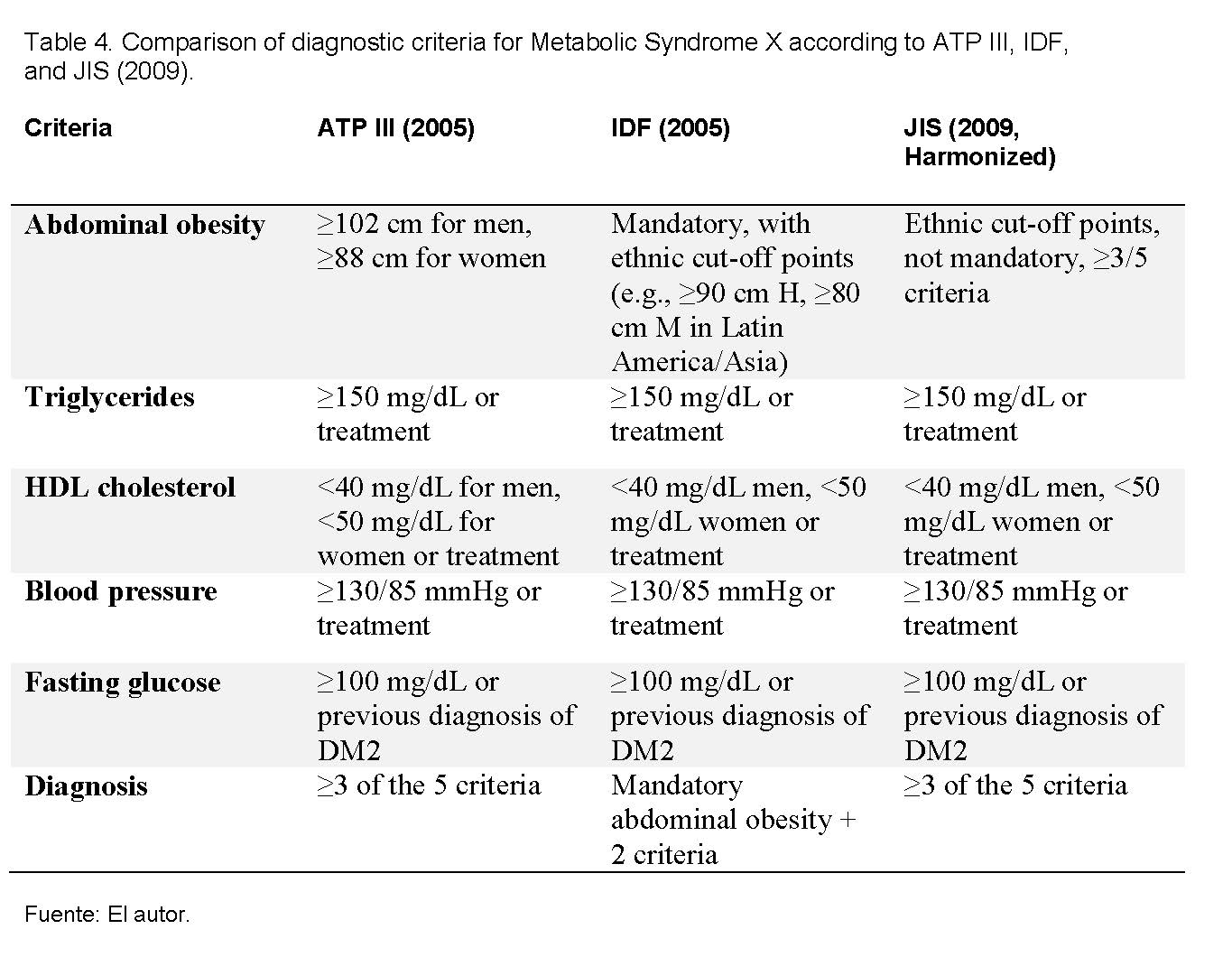
The study universe consisted of the population of the province of Colón (127,582 inhabitants), and the sample included all patients treated with dialysis for chronic renal failure (CRF) during the year 2005. All patients on hemodialysis or peritoneal dialysis in the Hemodialysis Unit at Amador Guerrero Hospital with a previous or established diagnosis of CRF during the year 2005 were included, and those who entered after 2006 or had undergone a kidney transplant were excluded. Data were collected over a three-month period, covering both hospital unit patients and private hemodialysis units in the province. After obtaining informed consent, anthropometric parameters such as dry weight, abdominal circumference, and height were recorded, taking averages of the measurements. Laboratory data corresponding to the months of January, June, and December were extracted from clinical records, including age, fasting glucose, blood pressure, and lipid profile (total cholesterol, LDL, HDL, and triglycerides). For the diagnosis of metabolic syndrome X, the NCEP/ATP III (2005) criteria were used, which establish the presence of at least three of the following factors: abdominal obesity (>120 cm in men and >80 cm in women), hypertriglyceridemia (>150 mg/dl), low HDL cholesterol (<40 mg/dl in men and <50 mg/dl in women), hypertension (≥130/85 mmHg), and elevated fasting glucose (≥100 mg/dl). Finally, the data were tabulated in Microsoft Excel and subsequently exported to EPI-INFO 6.0 for statistical analysis and significance testing.
RESULTS
The studied population of 38 patients consisted of patients already diagnosed with CRF, mostly in the age group of 30 to 59 (See Table 1). This group represented 52.6% of the cases. This was followed by the group of 18 to 39 years, representing 31.6% of the studied group. Finally, the group over 60 years represented 15.8% of the studied cases.
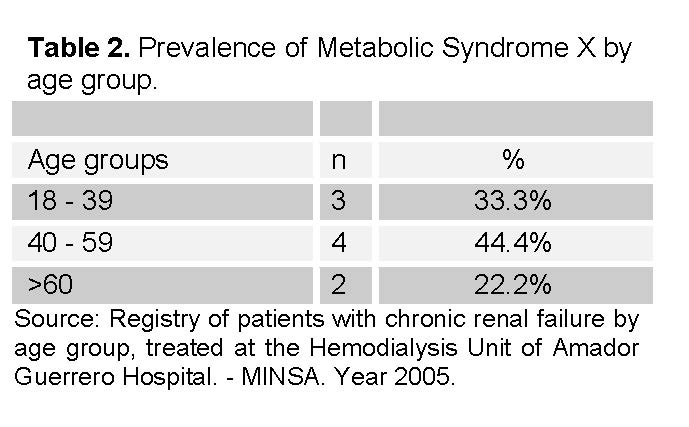
Of the 38 patients studied, 9 were diagnosed with MSX (See Table 2), therefore, 29 patients did not present MSX. Regarding the prevalence of MSX, it is also observed that there was a majority of MSX cases in the group of 40 to 59, with a total of 4 cases, followed by the age groups of 18 to 39 (3 cases) and the age group over 60 (2 cases).
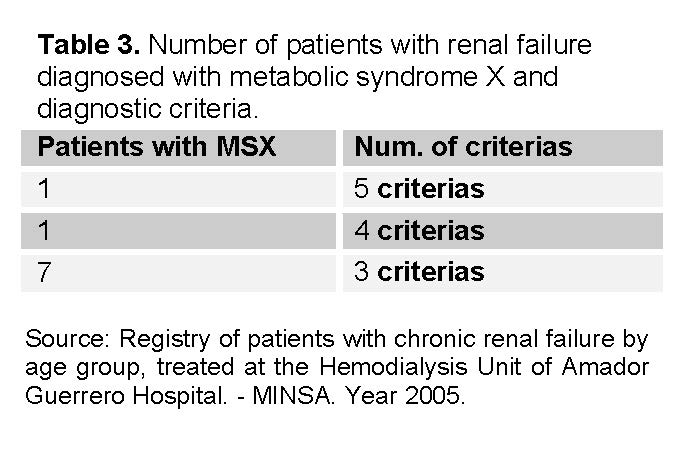
Regarding compliance with the diagnostic criteria for MSX, the 9 patients presented diagnostic criteria for MSX, of which the majority (7 cases) met 3 different criteria (See table 3 and 4). Only two patients met 4 and 5 criteria respectively.
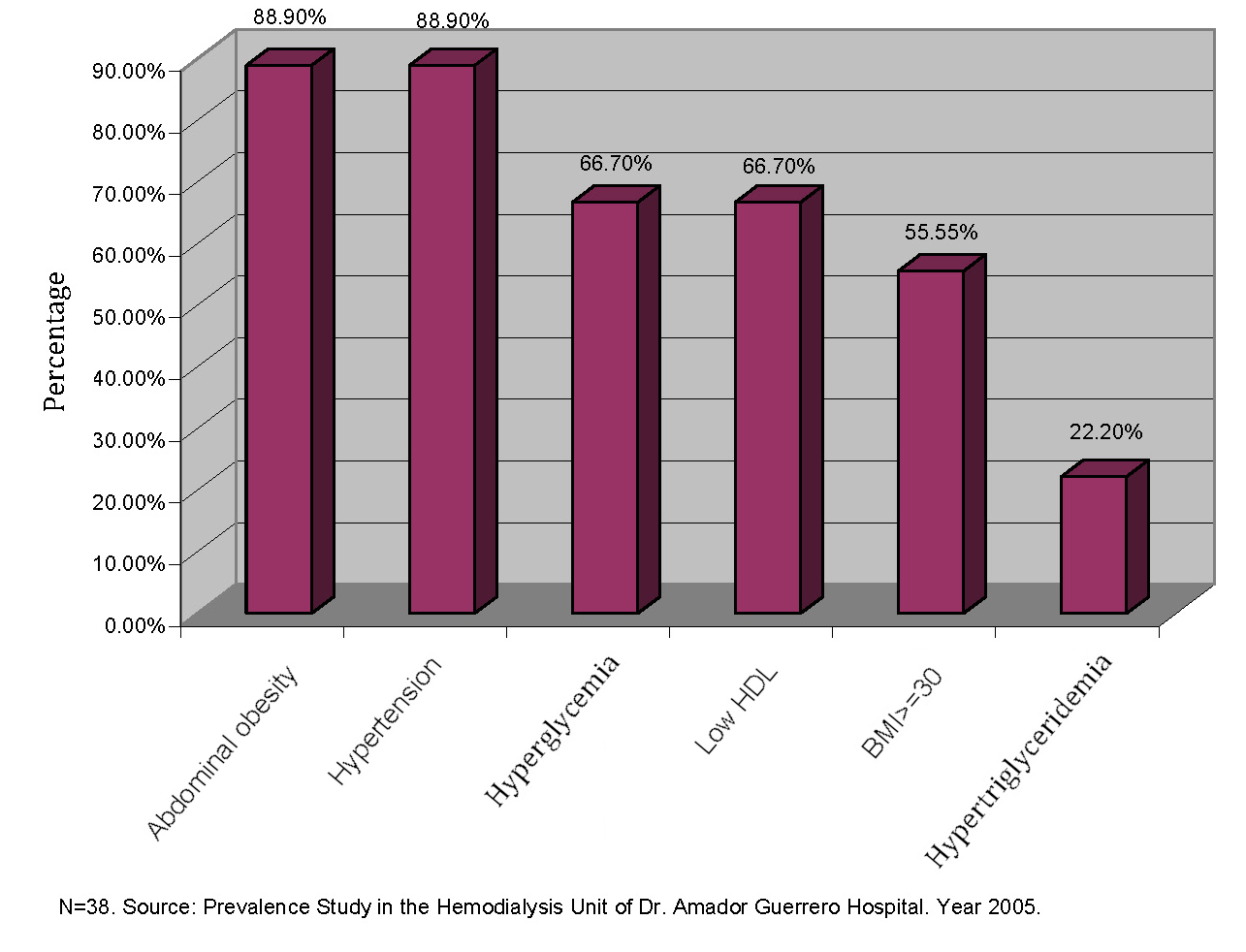
The 29 patients without MSX diagnoses were also evaluated regarding the presence of factors associated with MSX. Among the most frequent factors, it is observed in Figure 1 that abdominal obesity and hypertension occupy the first place with a prevalence of 88.9% both. Subsequently, insulin resistance measured by fasting hyperglycemia; then low HDL levels, and hypertriglyceridemia were less frequent factors.
Statistical Significance
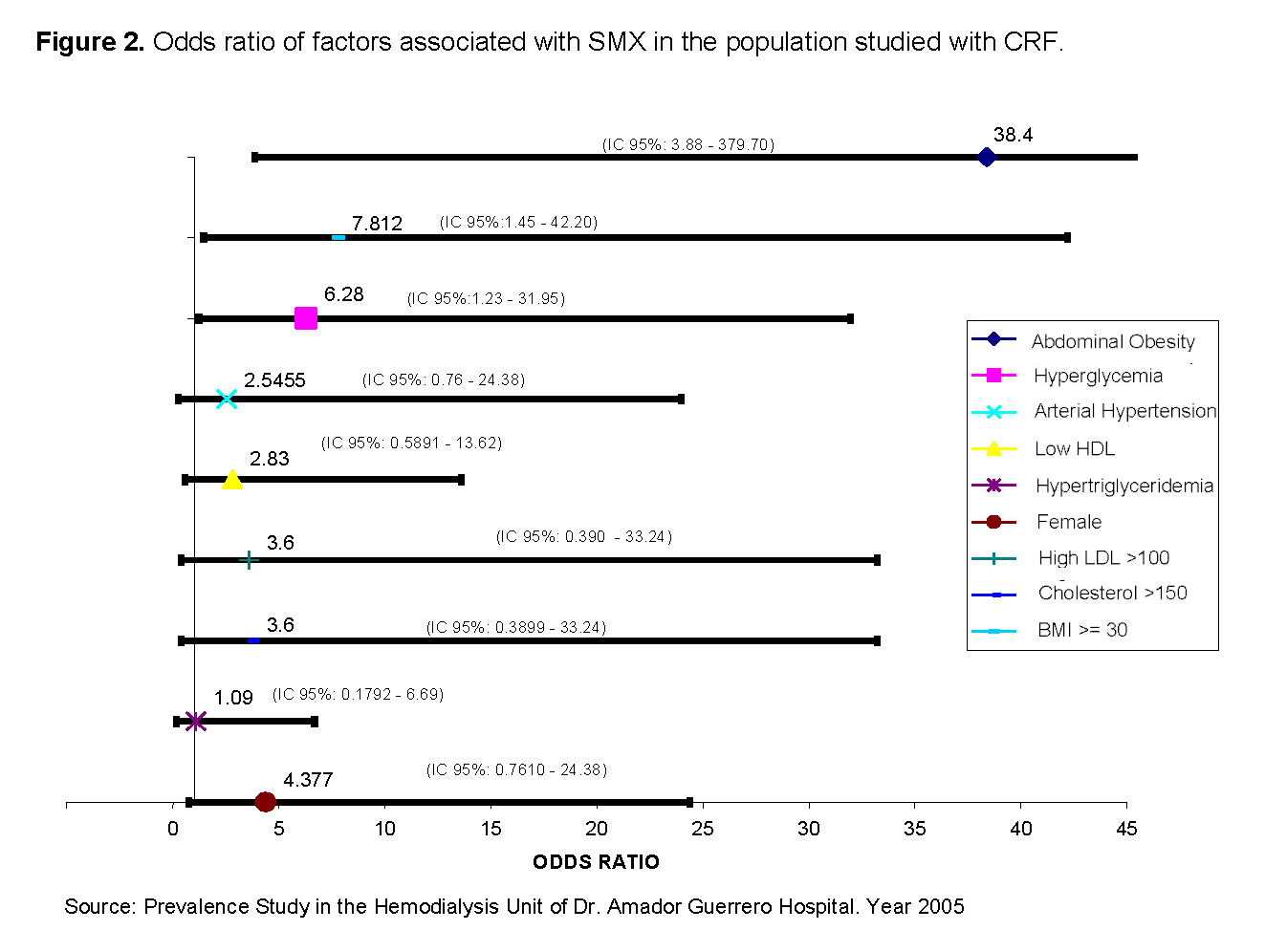
The risk factors were evaluated in the 38 patients. Abdominal obesity and hyperglycemia met the statistical analysis of X2 >3.86. 95% Confidence Interval with a p <0.05 (See Figure 2). By giving a statistical treatment to the variables under study, we could observe that abdominal obesity, body mass index, and hyperglycemia were the elements directly associated with Odds ratio of 38.4 (X2>3.86 IC 95%(3.88 – 379.70, p<0.05)), 7.8 8 (X2>6.26, IC 95% (1.45 – 42.20), p<0.05), and 6.3 (X2>6.26 IC 95%(1.23 – 31.95, p<0.05)) respectively.
DISCUSSION
Metabolic syndrome X (MSX) represents a constellation of metabolic alterations that include abdominal obesity, insulin resistance, hypertension, and dyslipidemia, all linked to an increased risk of type 2 diabetes and cardiovascular disease. Chronic renal failure (CRF), on the other hand, constitutes a complex clinical state characterized by the progressive loss of renal function and a high burden of metabolic comorbidities [1,2,4].
It should be noted that in 2023 the American Heart Association (AHA) introduced the term “cardiovascular-kidney-metabolic syndrome” (CKM) to highlight not only the relevance of diabetes but also the cardiovascular and renal components [10]. Likewise, some authors have suggested expanding the classic criteria of metabolic syndrome to include hyperuricemia and metabolic dysfunction-associated steatotic liver disease (MASLD) [11]. In this sense, it is pertinent to clarify that the present work refers to metabolic syndrome in its traditional conception; however, the results obtained seem to support the idea of a re-evaluation of the criteria towards the definition of cardiovascular-metabolic syndrome (CKM), which more comprehensively reflects the interaction between metabolic, cardiovascular, and renal factors.
The interconnection between both conditions is close: chronic kidney disease (CKD) generates a chronic inflammatory state, insulin resistance, and endothelial dysfunction, which favors the development of metabolic syndrome (MSX); in turn, the presence of MSX accelerates the progression of renal damage and increases cardiovascular risk in these patients [12,13]. In this study, the diagnostic criteria of NCEP/ATP III (2005) were applied, widely used at the time and considered a valid reference to assess the prevalence of risk factors in the analyzed population [14]. Although more recent criteria such as those of the IDF (2005) and the Joint Interim Statement (2009) have refined the cut-off points —especially those of waist circumference adjusted by ethnicity— [15,16], the findings of this work remain relevant to understand the magnitude of MSX in patients with CKD and serve as a comparative basis for future research. The Body Mass Index (BMI) has been included since, although it is not one of the diagnostic criteria for MSX, it is widely used in other studies and represents an important risk factor.

Regarding the age of patients with CRF and MSX, it is noteworthy that most cases, both of CRF and MSX, were in the group of 40 to 59 years, with a prevalence of 52.6% and 44.4% respectively. Although a larger case series is required to show statistical relevance, a trend can be observed in this age group. This also suggests that these conditions may be underdiagnosed in previous age groups. In contrast, it is expected that the group over 60 years has a lower prevalence given the nature of the disease and the relatively short survival [17].
Of the 9 patients with MSX, we observed that 5 had a BMI greater than or equal to 30Kg/m2. This represents 55.5% of the group. On the other hand, hyperglycemia was observed in 66.6% of the cases of patients diagnosed with MSX. Abdominal obesity was the most prevalent factor in this group, representing 88.8% of the cases diagnosed with MSX.
In the entire group of 38 patients studied, abdominal obesity and hypertension were the main associated factors, present in 88.9% of the patients. This was followed by the presence of hyperglycemia and low HDL levels in 66.7% of the patients. The body mass index, although not part of the diagnostic criteria for MSX, was included, being present in 55.5% of the cases studied. Hypertriglyceridemia was one of the least frequent factors, appearing in 22.2% of the cases. Similar to what is found in the scientific literature, abdominal obesity and hyperglycemia are factors frequently associated with the presence of MSX [7,18].
Abdominal Obesity and Hyperglycemia met the statistical analysis of X2 >3.86. 95% Confidence Interval with a p <0.05. BMI≥30 was also significant, however, we did not take it as a diagnostic criterion in the study.
When evaluating these variables and their association with CRF, we could observe that both abdominal obesity, body mass index, and hyperglycemia represented positive OR values with statistical significance. The most important factor was abdominal obesity with an OR of 38.4, followed by body mass index with an OR of 7.8; and finally, hyperglycemia with an OR of 6.3. It is important to highlight the increased probability shown by abdominal obesity measured by the OR when compared to the other factors evaluated.
The results obtained show a clear hierarchy in the strength of association of the factors studied with the presence of metabolic syndrome X (MSX). Abdominal obesity presented an OR of 38.4, meaning that patients with this finding are 38 times more likely to develop MSX compared to those without abdominal obesity. This extremely high value highlights abdominal obesity as the most important and determining risk factor in this cohort of patients with chronic renal failure (CRF).
The body mass index (BMI) ≥30 kg/m² was also significantly associated with MSX, showing an OR of 7.8. This indicates that obese patients are almost eight times more likely to present the syndrome. However, its strength of association is considerably lower than that of abdominal obesity, suggesting that the distribution of body fat (particularly visceral fat) plays a more relevant role than general excess weight in the development of MSX.
For its part, fasting hyperglycemia showed an OR of 6.3, indicating that patients with elevated glucose have six times more likely to present the syndrome. Although this result is clinically relevant, its impact is less than that of abdominal obesity, reaffirming that insulin resistance is a key component, but not the main determinant in this population.
Together, these findings allow us to conclude that, in patients with CKD, abdominal obesity is the strongest predictor of metabolic syndrome, followed by general obesity and hyperglycemia. This has important clinical implications, as it underscores the value of abdominal circumference as a predictive and risk stratification tool, even more so than BMI. It also reinforces the need for prevention programs aimed at controlling central obesity and strict monitoring of fasting glucose, with the goal of reducing cardiovascular risk and improving the clinical outcomes of renal patients.
Upon reviewing recent literature, we found that other research groups have also made associations and observations of hemodialysis patients and cardiovascular events associated with Metabolic Syndrome X [19], documenting a doubled risk of hospitalizations for cardiovascular events in those diagnosed with MSX. Interestingly, another group from Spain also conducted a literature review of publications on renal patients with Metabolic Syndrome X, in which it is observed that body mass index, insulin resistance, arterial hypertension, obesity, and lipid alterations are aspects that not only the doctor should consider, but also the nursing team attending to these patients [20].
Finally, this study does not include genetic factors, but it is known that there is a genetic predisposition associated with both CKD and MSX.
Although it is recognized that external factors such as a sedentary lifestyle, inadequate diet, overweight, and quality of life are determinants in the onset of metabolic syndrome X (MSX), evidence suggests that genetic factors also play a fundamental role in the development of this constellation of clinical conditions. MSX is not limited to the simple coexistence of abdominal obesity, arterial hypertension, dyslipidemia, and insulin resistance, but reflects a complex interaction between environment and genetic predisposition, where individual variability in genes associated with energy metabolism, inflammation, and vascular regulation can explain why some patients with similar lifestyles have a greater susceptibility to developing the disease. In fact, evidence in the scientific literature has identified that polymorphisms in genes such as LEP, ADIPOQ, INSR, IRS1, TCF7L2, PPARG, APOE, LPL, CETP, ACE, AGT, NOS3, TNF, IL6, among others, contribute to the genetic susceptibility to MSX in renal patients (See Table 5) [9].
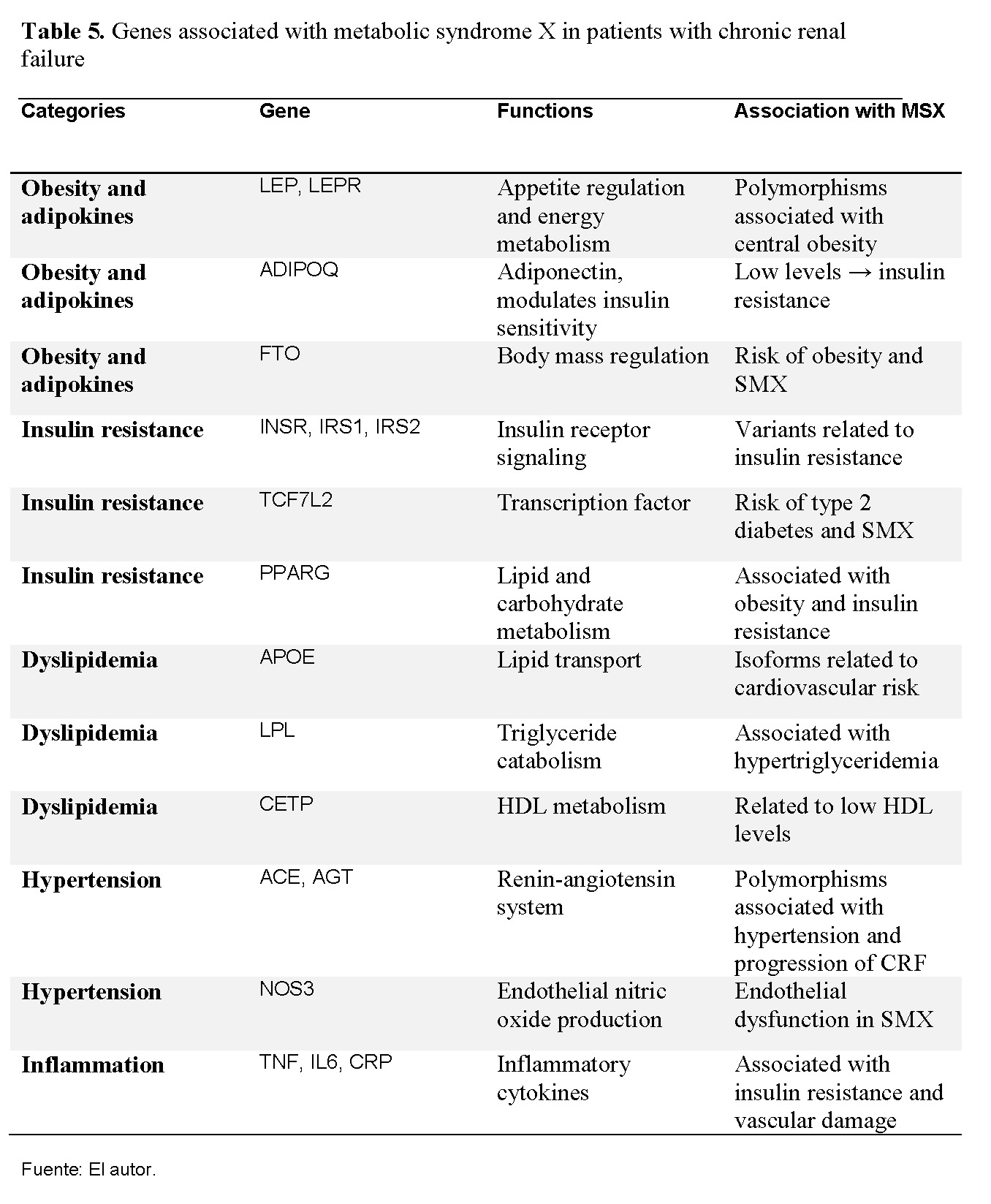
Polymorphisms in PPARG and ADIPOQ have been related to insulin resistance and abdominal obesity, while variants in ACE and AGT influence hypertension and CKD progression. Inflammatory genes such as TNF and IL6 contribute to a chronic inflammatory state that worsens insulin resistance and endothelial dysfunction [9]. This suggests that the interaction between genetics and environment explains the observed clinical variability.
CONCLUSIONS
Metabolic syndrome X (MSX) was present in 23.7% of patients with chronic kidney disease (CKD) on dialysis in the province of Colón, which indicates a high prevalence in this population.
The majority of cases were concentrated in the age group of 40 to 59 years, a productive stage of life, which implies significant social and economic consequences for the affected population.
The factors most strongly associated with MSX were abdominal obesity, body mass index ≥30 kg/m², and fasting hyperglycemia. Among them, abdominal obesity showed the greatest strength of association, indicating that visceral fat is the main clinical determinant of the syndrome in this cohort.
Although general obesity measured by BMI was associated with MSX, the risk was much greater when abdominal obesity was considered. This suggests that the distribution of body fat is more relevant than total excess weight in explaining metabolic risk in renal patients.
Other factors such as sex, low HDL levels, isolated arterial hypertension, and hypertriglyceridemia did not show a statistically significant association in this study, although their participation in the pathophysiology of the syndrome cannot be ruled out.
The interrelation between MSX and CKD is bidirectional: renal insufficiency promotes insulin resistance, chronic inflammation, and endothelial dysfunction, while MSX contributes to accelerating renal damage progression and increasing cardiovascular risk.
Various international studies have identified polymorphisms in genes such as LEP, ADIPOQ, PPARG, TCF7L2, INSR, IRS1, APOE, LPL, CETP, ACE, AGT, NOS3, TNF, and IL6 as factors that modulate genetic susceptibility to MSX in patients with kidney disease. These findings reinforce the importance of the interaction between genetic predisposition and environmental factors in the clinical expression of the disease.
Modern molecular techniques, such as comparative genomic hybridization (CGH) and targeted panel massive sequencing (NGS), represent valuable tools for identifying risk variants. Their application could allow personalized diagnoses and early interventions in subgroups of patients with genetic predisposition.
Although this study used the diagnostic criteria of the NCEP/ATP III (2005), different from more recent consensuses such as those of the IDF and JIS (2009), the results offer a valid basis for evaluating the prevalence of risk factors in this population and constitute a useful historical reference for future comparisons.
Recommendations
Develop preventive programs focused on controlling abdominal obesity through education on healthy lifestyles.
Promote strict monitoring of insulin resistance, facilitating access to devices for self-monitoring of capillary glucose.
Conduct multicenter studies in Panama to evaluate the health condition of patients with CKD and relevant risk factors.
Integrate the analysis of genetic polymorphisms associated with MSX in future research to identify subgroups with greater susceptibility.
Acknowledgments
Thanks to Dr. Alexander Ballesteros, Panamanian nephrologist, for his review and substantial recommendations to the manuscript. We also thank Dr. Miguel Santana, who during his Head of Teaching at the Manuel Amador Guerrero Hospital reviewed and approved the study.
References
[1] Zillich AJ, et al. Caring for Patients With Chronic Kidney Disease. Pharmacotherapy. 2005;25(1):123-143.
[2] Hanson RL, et al. Components of the Metabolic Syndrome and Incidence of Type 2 Diabetes. Diabetes. 2002;51(10):3120-3127.
[3] Ford ES, et al. Increasing Prevalence of the Metabolic Syndrome Among U.S. Adults. Diabetes Care. 2004;27(10):2444-2449.
[4] Grundy SM, et al. Definition of Metabolic Syndrome. Circulation. 2004;109:433-438.
[5] OPS. Informe de Indicadores de Salud, Panamá. [2003]
[6] Contraloría General de la Nación. Panamá en Cifras. [2004]
[7] Thomas G, et al. Metabolic syndrome and kidney disease: a systematic review. CJASN. 2011;6(10):2364–73.
[8] Kovesdy CP, et al. Obesity and kidney disease: Hidden consequences. Kidney Int. 2017;91(2):260–2.
[9] Gu J, et al. Genetic polymorphisms in metabolic syndrome and chronic kidney disease. Kidney Blood Press Res. 2018;43:1312–21.
[10] Visseren FLJ, Perkovic V, Arnett DK, et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory From the American Heart Association. Circulation. 2023;148(16):e331–e350. doi:10.1161/CIR.0000000000001170.
[11] Rinella ME, Lazarus JV, Ratziu V, et al. A multi-society Delphi consensus on new terminology for fatty liver disease. J Hepatol. 2023;79(5):1149–1162. doi:10.1016/j.jhep.2023.07.025.
[12] Verdalles Guzman, Ursula et. al, Calcifilaxis: complicación grave del síndrome cardio-metabólico en pacientes con enfermedad renal crónica terminal (ERCT), Nefrologia, 2008;28(1):1-121. Disponible en: https://www.revistanefrologia.com/es-calcifilaxis-complicacion-grave-del-sindrome-articulo-X0211699508033201?referer=buscador
[13] Gu J, et al. Genetic polymorphisms in metabolic syndrome and chronic kidney disease. Kidney Blood Press Res. 2018;43:1312–21.
[14] Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive Summary of The Third Report of the National Cholesterol Education Program (NCEP) Expert Panel. JAMA. 2001;285(19):2486–2497.
[15] International Diabetes Federation. IDF Consensus Worldwide Definition of the Metabolic Syndrome. [2005]
[16] Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; et al. Circulation. 2009;120(16):1640–1645. doi:10.1161/CIRCULATIONAHA.109.192644.
[17] Roldan Torres, Ildefonso, et. al., Pronóstico a largo plazo de la enfermedad renal crónica en el síndrome coronario agudo sin elevación del segmento ST tratado con estrategia invasiva, Nefrologia 2017:37:(3):276-284. DOI: https://doi.org/10.1016/j.nefro.2016.11.011
[18] O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look. Obes Rev. 2015;16:1–12.
[19] Pérez de José Ana, Verdalles-Guzmán Úrsula, Abad Soraya, Vega Almudena, Reque Javier, Panizo Nayara et al . El síndrome metabólico se asocia con eventos cardiovasculares en hemodiálisis. Nefrología (Madr.) [Internet]. 2014 [citado 2025 Sep 04] ; 34( 1 ): 69-75. Disponible en: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0211-69952014000100009&lng=es. https://dx.doi.org/10.3265/Nefrologia.pre2013.Oct.12092.
[20] Andreu i Periz Dolores, Hidalgo Blanco Miguel Angel, Moreno Arroyo Carmen. El síndrome metabólico en el paciente renal. Enferm Nefrol [Internet]. 2014 Mar [citado 2025 Sep 04];17(1): 59-61. Disponible en: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S2254-28842014000100010&lng=es.
suscripcion
issnes
eISSN L 3072-9610 (English)
Information
Use of data and Privacy - Digital Adversiting
Infomedic International 2006-2025.

